Port Delivery System Genentech
Port delivery system genentech. Arshad Khanani MD director of clinical research at Sierra Eye Associates presented data from the LADDER phase 2 trial at this years conference. Image from Genentech Genentech announced that the FDA accepted its Biologics License Application BLA for its Port Delivery System PDS with ranibizumab. SOUTH SAN FRANCISCO Calif June 24 2021--Genentech a member of the Roche Group SIX.
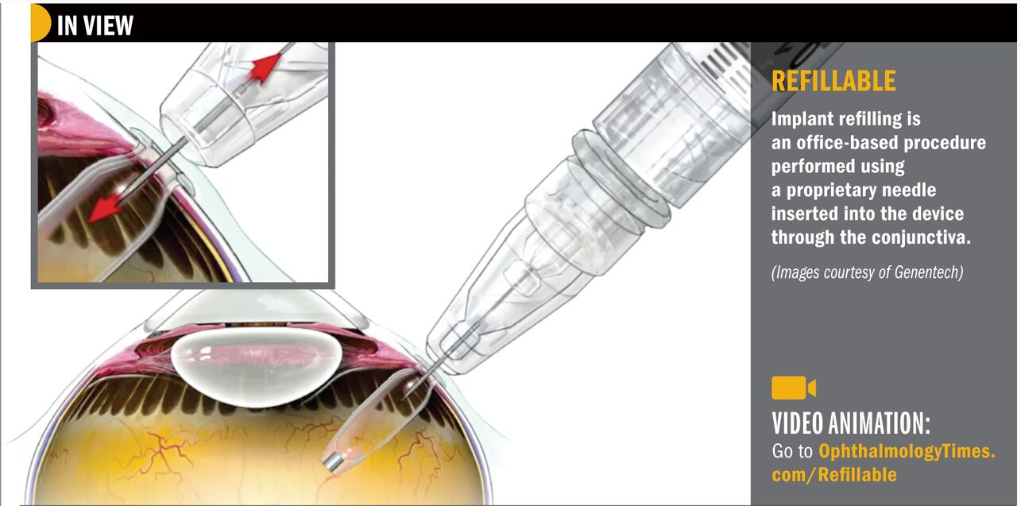
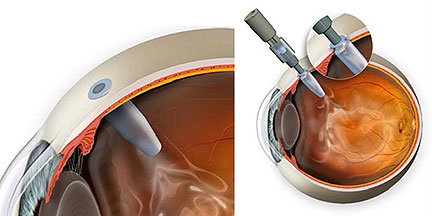
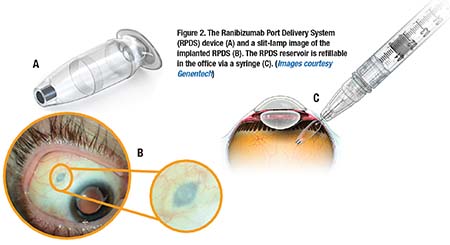
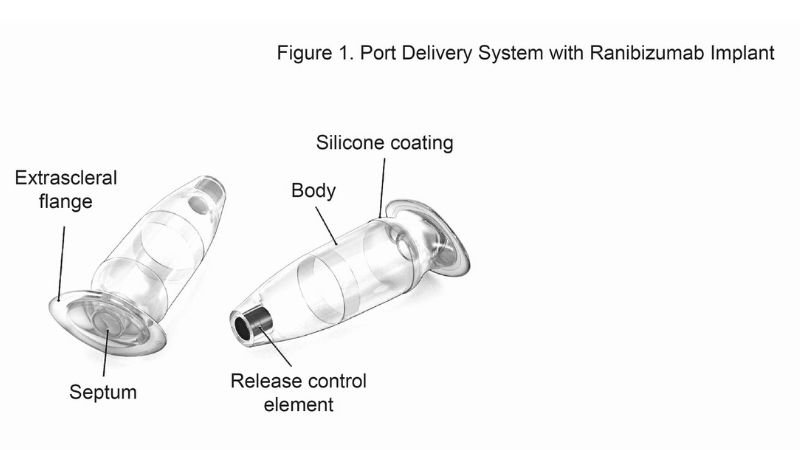
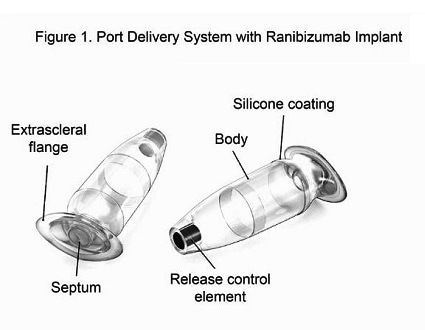
The PDS is a permanent refillable eye implant thats the approximate size of a grain of rice. The PDS provides a continuous delivery of a customized ranibizumab formation. A New Port Delivery System for Ranibizumab No not that kind of port.
The Port Delivery System with ranibizumab has been FDA approved for use in patients with neovascular age-related macular degeneration in patients who previously responded to at. The Port Delivery System with ranibizumab PDS from Genentech was one of those potential novel therapies. FDA Accepts Genentechs BLA for Port Delivery System with Ranibizumab for Wet AMD.
But just recently at the end of June the FDA accepted Genentechs biologics license application for its port delivery system PDS of ranibizumab. The Port Delivery System PDS with ranibizumab Lucentis Genentech Inc an investigational drug delivery system designed for continuous long-term intravitreal drug delivery to treat neovascular age-related macular degeneration nAMD was found to be safe and efficacious in the phase 3 Archway trial which evaluated fixed refill exchanges done every 24 weeks compared with monthly. Port Delivery System with ranibizumab PDS is a permanent refillable eye implant approximately the size of a grain of rice which is designed to.
Genentechs parent company Roche is investigating a bispecific antibody for the treatment of retinal eye diseases. About Genentech Founded more than 40 years ago Genentech is a leading biotechnology company that discovers develops manufactures and. Phase 2 multicenter randomized active treatment-controlled clinical trial.
RHHBY today announced that the US. To evaluate the safety and efficacy of the Port Delivery System with ranibizumab PDS for neovascular age-related macular degeneration nAMD treatment. Genentechs PDS implant.
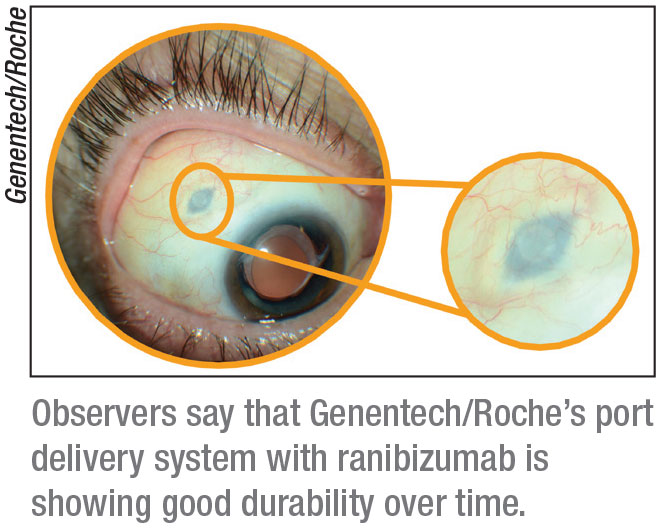
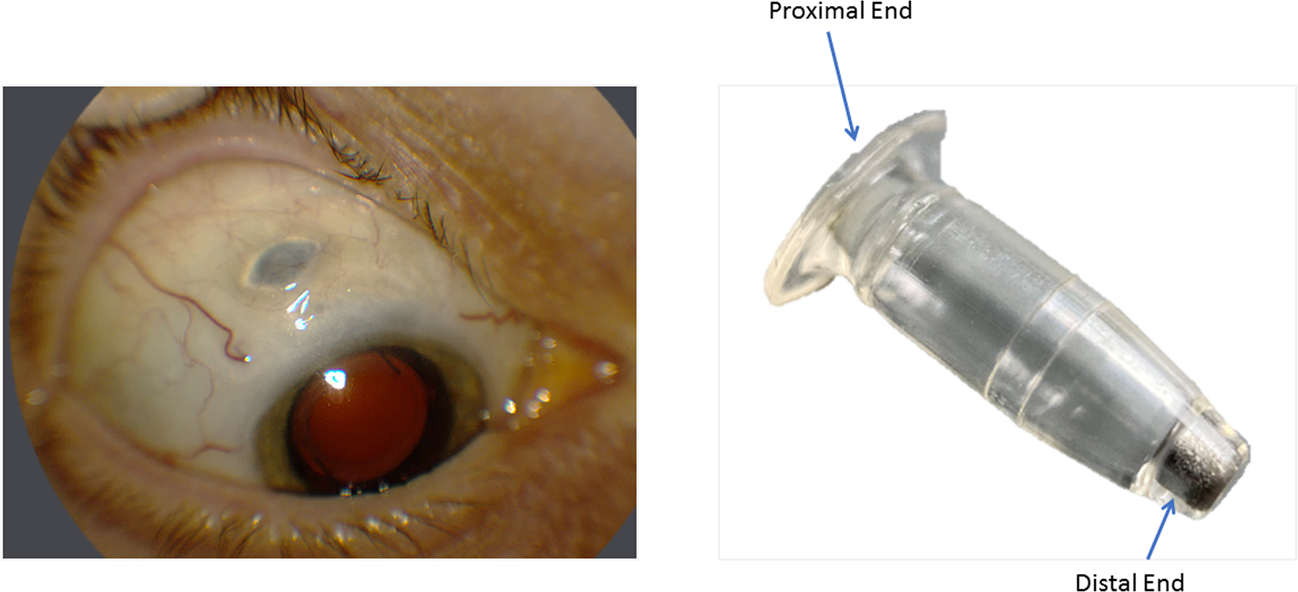
Phase III Archway trial data presented at the American Society of Retina Specialists showed the vast majority of patients984 percent to be precisewent six months before needing any additional treatment. Eye - The Port Delivery System with ranibizumabjourney of mitigating vitreous.
The Port Delivery System with ranibizumab has been FDA approved for use in patients with neovascular age-related macular degeneration in patients who previously responded to at.
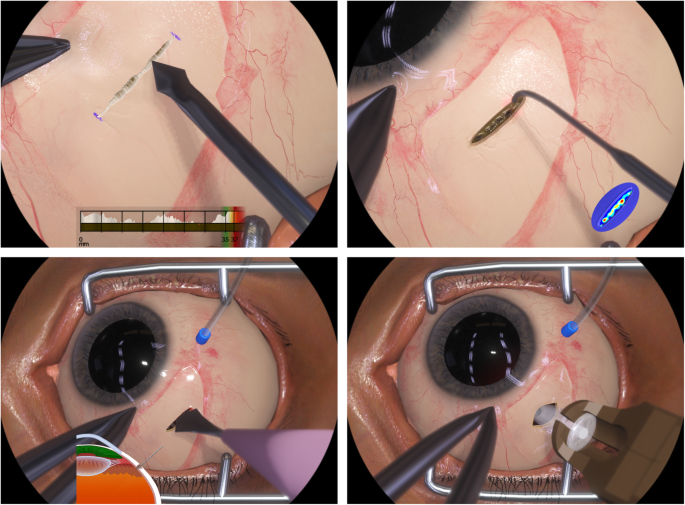
FDA Approves Genentechs Port Delivery System Susvimo a First-of-Its-Kind Therapeutic Approach for Wet AMD. Genentechs parent company Roche is investigating a bispecific antibody for the treatment of retinal eye diseases. Phase 2 multicenter randomized active treatment-controlled clinical trial. Arshad Khanani MD director of clinical research at Sierra Eye Associates presented data from the LADDER phase 2 trial at this years conference. Genentech has been working on a long-lasting port delivery system for ranibizumab for some time now. Phase III Archway trial data presented at the American Society of Retina Specialists showed the vast majority of patients984 percent to be precisewent six months before needing any additional treatment. FDA Accepts Genentechs BLA for Port Delivery System with Ranibizumab for Wet AMD. FDA Accepts Application for Genentechs Port Delivery System With Ranibizumab PDS for Treatment of Wet Age-Related Macular June 24 2021 100 AM EDT SHARE THIS ARTICLE. The Port Delivery System PDS for ranibizumab Genentech aims to combat these issues through the implantation of a permanent intraocular reservoir that is refillable via an in-office procedure.
The Port Delivery System with ranibizumab PDS from Genentech was one of those potential novel therapies. Phase 2 multicenter randomized active treatment-controlled clinical trial. About Genentech Founded more than 40 years ago Genentech is a leading biotechnology company that discovers develops manufactures and. FDA Accepts Application for Genentechs Port Delivery System With Ranibizumab PDS for Treatment of Wet Age-Related Macular June 24 2021 100 AM EDT SHARE THIS ARTICLE. Port Delivery System with ranibizumab PDS is a permanent refillable eye implant that continuously delivers ranibizumab over a period of months potentially reducing the treatment burden associated with frequent eye injections. Arshad Khanani MD director of clinical research at Sierra Eye Associates presented data from the LADDER phase 2 trial at this years conference. The Port Delivery System with ranibizumab PDS has been the focus of much attention in the late summer.




























Post a Comment for "Port Delivery System Genentech"